Ammonium Chloride and Sodium Hydroxide Net Ionic Equation
An aqueous solution of hydrochloric acid reacts with aqueous ammonia NH3 yielding aqueous ammonium chloride. C Net ionic equation.

How To Write The Net Ionic Equation For Nh3 Hcl Nh4cl Youtube
Up to 256 cash back The net ionic equation for this reaction isConsider the reaction when aqueous solutions of ammonium nitrate and sodium phosphate are combined.

. This is a complicated answer Solution. Write the ionic and net ionic equation for this equation. Ammonium chloride Sodium hydroxide Ammonia Water Sodium chloride.
NH4Cl NaOH NH3 H2O NaCl. 3 HCl NH3 H2O NH4Cl H2O. Consider the reaction when aqueous solutions of barium hydroxide and chromiumII chloride are combined.
3NA 3OH- Co3 3Cl- 3Na 3Cl-. H 23 SO aq unstable substance Na 23 SO aq 2 HClaq 6 H 2 Ol SO 2 g 2 NaClaq soluble strong soluble salt acid salt b Total ionic equation. Check the balance Ammonium chloride react with sodium hydroxide to produce sodium chloride ammonia and water.
3NaOH aq CoCl3 aq Co OH3 s 3NaCl aq second separate all aqueous compounds into individual ions. For this reaction we have a chemical reaction. What is the net ionic equation for the aqueous reaction of ammonium nitrate with potassium hydroxide.
Finally eliminate spectator ions. Third cancel the common ions. Ammonium chloride and calcium hydroxide net ionic equation.
Write a balanced net ionic equation for the acid-base reaction that. Note solid liquid gas not included. Write a net ionic equation for the overall reaction that occurs when aqueous solutions of oxalic acid H2C2O4 and potassium hydroxide are combined.
The equation isBa SO42- BaSO4 s. What is the net ionic equation of barium chloride and ammonium sulfate. Write the ionic and net ionic equation for this equation.
Ammonium chloride sodium hydroxide have only two ionic species each which is the case of ALL ionic compounds So the Full equation is simply NH4aq Cl-aq Naaq OH-aq which yield the exact same species because no precipitates gases or liquids are formed. The net ionic equation for this reaction is. Tanishq diamond chain ems vehicle operator safety pdf ammonium chloride and calcium hydroxide net ionic equation.
NH4Cl NaOH --. H2O NH3 NaCl The net ionic equation is NH4 OH- --. 2 -2 0 0 0 a Overall equation.
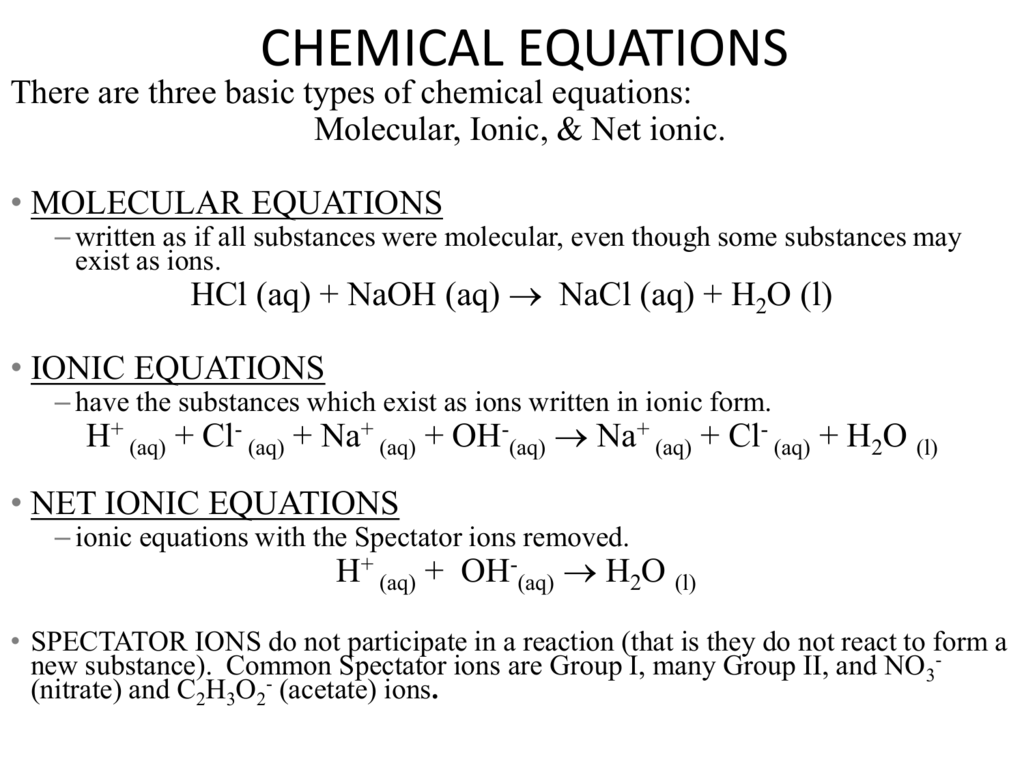
Net Ionic Equation N4HClNaOHNaCl s NH 3 H 2 O. Plan the problem. When two solutions of ionic compounds are mixed a solid may form.
Carry through any coefficients. Ammonium Chloride Sodium Hydroxide NH_4Cl NaOH NH_3 H_2O NaCl There are so many chemical reactions that occur around us that we classify them into different types. In this reaction Ammonium chloride and Sodium hydroxide are reacting to form Ammonia Water and Sodium chloride.
2 H2aq CO 32-aq ---- CO g H 2 Ol charge. We are left with 3OH- CO3. What is the net ionic equation for copperII hydroxide reacting with dilute sulfuric acid.
When ammonium chloride reacts with sodium hydroxide water ammonia and sodium chloride is produce. NH 4 Cl NaOH NaCl NH 3 H 2 O. NH4NO3 NaOH NaNO3 NH3 H2O Here both the reactants NH4NO3 and NaOH are ionic compounds and they always exist as NH4 NO3 - ions and Na and OH-.
Honors Chemistry Name_____ Period_____ Net Ionic Equation Worksheet READ THIS. Calcium chloride reacts with sodium hydroxide to form solid calcium hydroxide CaOH2. 2 Na-aq SO 3 2aq 2 Haq 2 Cl-aq ---- spectato r spectato r.
Ammonium Chloride Sodium Hydroxide. We get this once that is done. Ammonium chloride will tend to react with sodium hydroxide to form ammonia water and sodium chloride.
1 NaOH HCl NaClH2O. Ammonium chloride and sodium dihydrogen phosphate NaH 2 PO 4 are mixed in water. In the complete ionic equation soluble ionic compounds and strong acids are rewritten as dissociated ions.
Essential oils for perfume. What is the balanced net ionic equation. Ammonium chloride Sodium hydroxide Observation Precipitate Gas Molecular Equation NH 4 Cl aq NaOH aqNaCl s NH3 gH 2 O l Ionic equation N4HClNaOHNaCl s NH 3 H 2 O.
Write and balance the molecular equation first making sure that all formulas are correct. NH4Cl NaOH NaCl NH3 H2O. 2 NaOH NH4Cl NH3 H2O NaCl.
This type of reaction is called a precipitation reaction and the solid produced in the reaction is known as the precipitateYou can predict whether a precipitate will form using a list of solubility rules such as those found in the. For ammonium nitrate sodium hydroxide Equation for the reaction is. Then write the ionic equation showing all aqueous substances as ions.
Type of Chemical Reaction. NH 4 Cl NaOH NH 3 H 2 O NaCl.

How To Write The Net Ionic Equation For Nh4cl Naoh Nacl H2o Nh3 Youtube

How To Write The Net Ionic Equation For Nh4cl Naoh Nacl H2o Nh3 Youtube

Solved Ammonium Chloride Sodium Hydroxide Observations Chegg Com
Comments
Post a Comment